What Happens To Mass And Energy During Physical And Chemical Changes
3.ix: Energy and Chemic and Physical Change
- Page ID
- 97989
Learning Objectives
- Define endothermic and exothermic reactions.
- Describe how rut is transferred in endothermic and exothermic reactions.
- Decide whether a reaction is endothermic or exothermic through observations, temperature changes, or an energy diagram.
So far, nosotros have talked about how energy exists as either kinetic free energy or potential energy and how energy can be transferred every bit either heat or work. While it'south important to sympathize the deviation betwixt kinetic energy and potential energy and the deviation between heat and work, the truth is, energy is constantly changing. Kinetic energy is constantly existence turned into potential energy, and potential free energy is constantly beingness turned into kinetic energy. Likewise, energy that is transferred as piece of work might after end up transferred as heat, while energy that is transferred as estrus might later end upwards being used to do work.
Fifty-fifty though energy tin can change form, it must still follow one fundamental law: Energy cannot be created or destroyed, it tin can but exist inverse from i form to some other. This constabulary is known as the Law of Conservation of Free energy. In a lot of ways, energy is similar money. You tin substitution quarters for dollar bills and dollar bills for quarters, but no thing how often you convert between the ii, y'all will not end up with any more or any less money than you started with. Similarly, y'all tin transfer (or spend) money using cash, or transfer coin using a credit card; but y'all still spend the aforementioned amount of money, and the store all the same makes the aforementioned amount of money.
A bivouac is an instance of basic thermochemistry. The reaction is initiated by the application of estrus from a lucifer. The reaction converting wood to carbon dioxide and water (among other things) continues, releasing oestrus energy in the process. This heat energy can then be used to melt food, roast marshmallows, or just keep warm when it's cold outside.

An prototype of a campfire with colored flames, made by the burning of a garden hose in a copper pipage. (CC SA-By 3.0; Jared)
Exothermic and Endothermic Processes
When physical or chemical changes occur, they are more often than not accompanied past a transfer of free energy. The police of conservation of energy states that in any concrete or chemical process, energy is neither created nor destroyed. In other words, the entire free energy in the universe is conserved. In order to ameliorate sympathize the free energy changes taking place during a reaction, we need to define ii parts of the universe:the system and the surroundings. The system is the specific portion of affair in a given space that is beingness studied during an experiment or an observation. The environs are everything in the universe that is not function of the system. In practical terms for a laboratory chemist, the organisation is the particular chemicals beingness reacted, while the environs are the immediate vicinity within the room. During virtually processes, energy is exchanged betwixt the system and the surroundings. If the organization loses a certain amount of energy, that aforementioned amount of energy is gained by the surroundings. If the system gains a certain amount of energy, that energy is supplied by the environment.
A chemic reaction or physical change is endothermic if heat is absorbed by the system from the surroundings. In the class of an endothermic process, the system gains heat from the surroundings and so the temperature of the surround decreases. The quantity of heat for a process is represented by the letter \(q\). The sign of \(q\) for an endothermic process is positive because the system is gaining heat. A chemical reaction or physical modify is exothermic if oestrus is released past the organization into the surroundings. Because the environment are gaining heat from the organisation, the temperature of the surroundings increases. The sign of \(q\) for an exothermic process is negative considering the arrangement is losing heat.
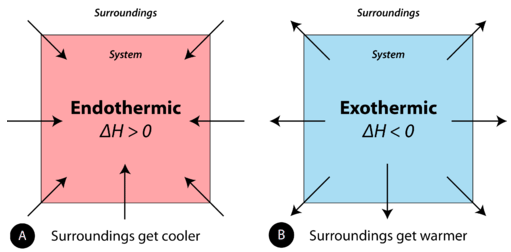
During phase changes, free energy changes are normally involved. For example, when solid dry water ice vaporizes (physical modify), carbon dioxide molecules blot energy. When liquid h2o becomes ice, free energy is released. Call back that all chemical reactions involve a alter in the bonds of the reactants. The bonds in the reactants are cleaved and the bonds of the products are formed. Chemic bonds have potential energy or "stored energy". Considering we are changing the bonding, this means we are also changing how much of this "stored energy" there is in a reaction.
Energy changes are frequently shown by drawing an energy diagram. Energy diagrams show the stored/hidden free energy of the reactants and products likewise as the activation free energy. If, on an energy diagram, the products take more stored energy than the reactants started with, the reaction is endothermic. You had to give the reaction free energy. If, on the energy diagram, the products have less stored energy than the reactants started with, the reaction is exothermic.
Example \(\PageIndex{one}\)
Label each of the following processes equally endothermic or exothermic.
- water boiling
- gasoline called-for
- ice forming on a swimming
Solution
- Endothermic—yous must put a pan of h2o on the stove and give it rut in club to become water to boil. Considering you are calculation rut/free energy, the reaction is endothermic.
- Exothermic—when you lot burn something, information technology feels hot to you because it is giving off heat into the surroundings.
- Exothermic—think of water ice forming in your freezer instead. Y'all put h2o into the freezer, which takes oestrus out of the water, to get information technology to freeze. Because heat is being pulled out of the water, it is exothermic. Heat is leaving.
Exercise \(\PageIndex{1}\)
Label each of the following processes as endothermic or exothermic.
- h2o vapor condensing
- gilded melting
- Answer (a)
- exothermic
- Answer (b)
- endothermic
Summary
Phase changes involve changes in energy. All chemic reactions involve changes in energy. This may be a change in heat, electricity, light, or other forms of free energy. Reactions that blot energy are endothermic. Reactions that release energy are exothermic.
Contributions & Attributions
This page was synthetic from content via the following contributor(southward) and edited (topically or extensively) by the LibreTexts development team to encounter platform fashion, presentation, and quality:
-
Marisa Alviar-Agnew (Sacramento City College)
-
Henry Agnew (UC Davis)
Source: https://chem.libretexts.org/Courses/College_of_Marin/CHEM_114%3A_Introductory_Chemistry/03%3A_Matter_and_Energy/3.09%3A_Energy_and_Chemical_and_Physical_Change
Posted by: nestorswilifewouse.blogspot.com

0 Response to "What Happens To Mass And Energy During Physical And Chemical Changes"
Post a Comment